Our team
PRZEMYSŁAW MAZUREK
President Of The Management Board
PIOTR MIERZEJEWSKI
Member Of The Management Board
TOMASZ CIACH
Member Of The Management Board
Supervisory Board
JERZY GARLICKI
ROBERT DZIUBŁOWSKI
STEFAN BOGUSŁAWSKI
Our development path
 |
DiscoveryOur dextran drug delivery system was first developed at the Faculty of Chemical and Process Engineering of the Warsaw University of Technology in the Biomedical Engineering Laboratory established by Dr. Tomasz Ciach. In 2013 GIZA Polish Ventures, together with Dr. Ciach decided to commercialize the technology and founded NanoVelos. Since then we work hard to broaden our portfolio of dextran-drug conjugates. In parallel, we explore new areas of interest eg. drug formulation development for lubrication of joints or sinusitis treatment. |
|||
 |
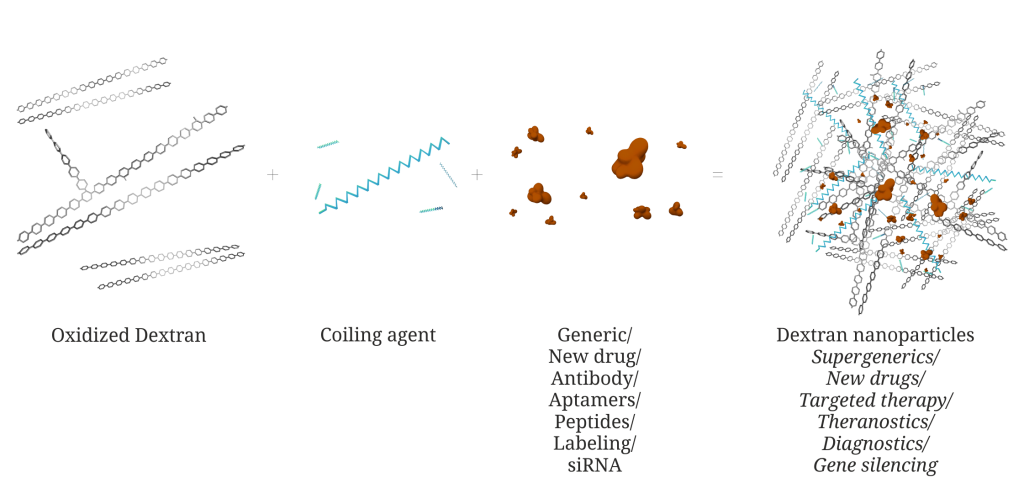
Our nanoparticlesare produced from various polysaccharides (e.g. from oxidized Dextran) without use of toxic substances or metal particles. Dextran can form a nanoparticles backbone composed of 10 modified dextran subunits, stabilized by the addition of coiling agents. Nanoparticles can serve as drug carriers with a pH-dependent drug bond that allows release of the drug solely within cancer cells. Technology based on polysaccharide nanoparticles is orginal solution patented by NanoVelos SA In vitro experiments: Assesment of cell viability (MTT assay, HeLa cells etc.) demonstrates that examined drugs released from nanoparticles retains its potential to induce cell death. |
|||
 |
Preclinical studiesNon-clinical studies form the basis for further clinical studies and drug development in humans. Usually this part consists of in-vitro (cell culture) and in-vivo (animals) studies of a new drug substance or drug carriers. The main goals of those studies is to collect the data providing submission to FDA or EMA. Preclinical studies as a first step of drug development process include: screening tests; tests on cell cultures; pharmacokinetics (single and multiple dose) and toxicity (MTD test) on healthy animals (mouse, rats, guinea pig); efficacy tests on animal models of human diseases. Preclinical studies must meet with EMA and FDA requirements and be done under GLP (Good Laboratory Practice) rules. GLP refers to a quality system of laboratories and ensure uniformity, consistency, quality and integrity of non-clinical studies starting from physicochemical analysis through the animal trials. The pharmacokinetics, toxicity and efficacy tests of dextran nanoparticles containing various drugs have been comprehensive studied. Our results indicate that several of our nanoparticles induced an important cytotoxicity in vitro, but showed decreased toxicity in vivo–evidenced and sufficient pharmacokinetics. |
|||
 |
Human clinical studiesThrough clinical trials, scientists and doctors determine safety and efficacy of new treatments. There are two main types of clinical studies: clinical trials and observational studies. Clinical trials involve human volunteers that is intended to develop medical knowledge how to prevent and detect cancer cells, how to improve the quality of life for patients during and after therapy. In a clinical trial, participants receive specific interventions according to the research plan or protocol prepared by the investigators. These interventions may be medical products, such as drugs or devices; procedures; or changes to participants’ behavior, such as diet. Clinical trials may compare a new medical approach to a standard one that is already available, to a placebo that contains no active ingredients, or to no intervention. When a new product or approach is being studied, it is not usually known whether it will be helpful, harmful, or no different than available alternatives. The investigators are obliged to determine the safety and efficacy of the intervention by measuring certain outcomes in the participants. To successfully register new cancer treatments some phases must be done: Phase 1 and 2: Exploratory study involving very limited human exposure to the drug. Researchers need to establish whether a new treatment is safe, what kind of side effects are, and the best dose of the new treatment. In oncological trials, in phase 1 studies also gather preliminary data on effectiveness for example slowing the tumor growth. In phase 3: investigators study whether the treatment works better than the current standard therapy, also compare the safety of the new treatment with current one. Phase 3 include larger numbers of participants to make sure that obtained results are reliable. Generally, in clinical trials is described also very early ‘phase 0’ and later ‘phase 4’. Phase 0 include very small trials to evaluate if a new agent should be tested in a phase 1 trial, whereas phase 4 study a long-term safety and effectiveness. This phase 4 is conducted after a new treatment has been approved and is on the market. |
|||
 |
FDA/EMA ApprovalThe EMA (European Medicines Agency), is the European Union’s equivalent to the U.S. Food and Drug Administration (FDA). If the EMA grants approval, the drug can be used throughout the European Union and in Iceland, Norway and Liechtenstein, FDA on the territory of the United States. Before new drug goes to market first companies have to run tests in laboratory and on different animals. In the end they bring that data to the EMA/FDA in the form of an Investigational New Drug application. If the EMA/FDA signs documents, the company starts testing the drug on humans. To see if the drug is safe and effective when used to treat or diagnose a disease. |
|||
 |
MarketEverything is in flux: markets, science and competitors change constantly, and a company attempting to bring a drug to market must stay on top of and roll with the dynamic landscape. Defining of our patient population is essential from a regulatory, clinical, payer and commercial perspective. It gives us an opportunity and risk trade-offs, and lends valuable insight to strategic decision-making and scenario planning. Treatments are not one-size-fits-all, that’s why we focus on various areas. |
Dextran nanoparticles
|
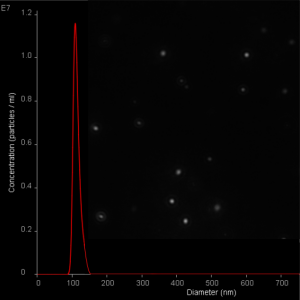
NanoSight
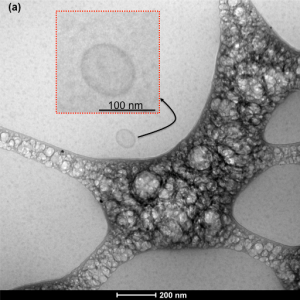
krioTEM
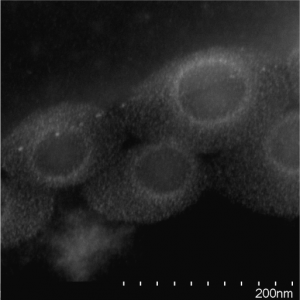
SEM with Ionic liquid (solvation of nanoparticles)
Goals
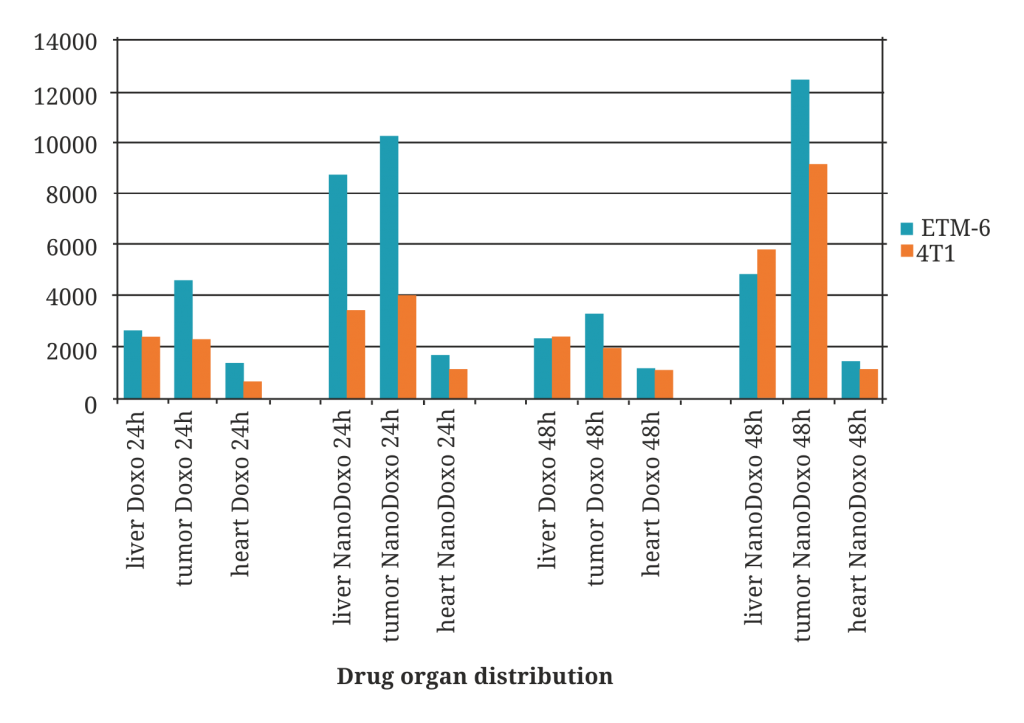 |
Widely proven targeting |
|||
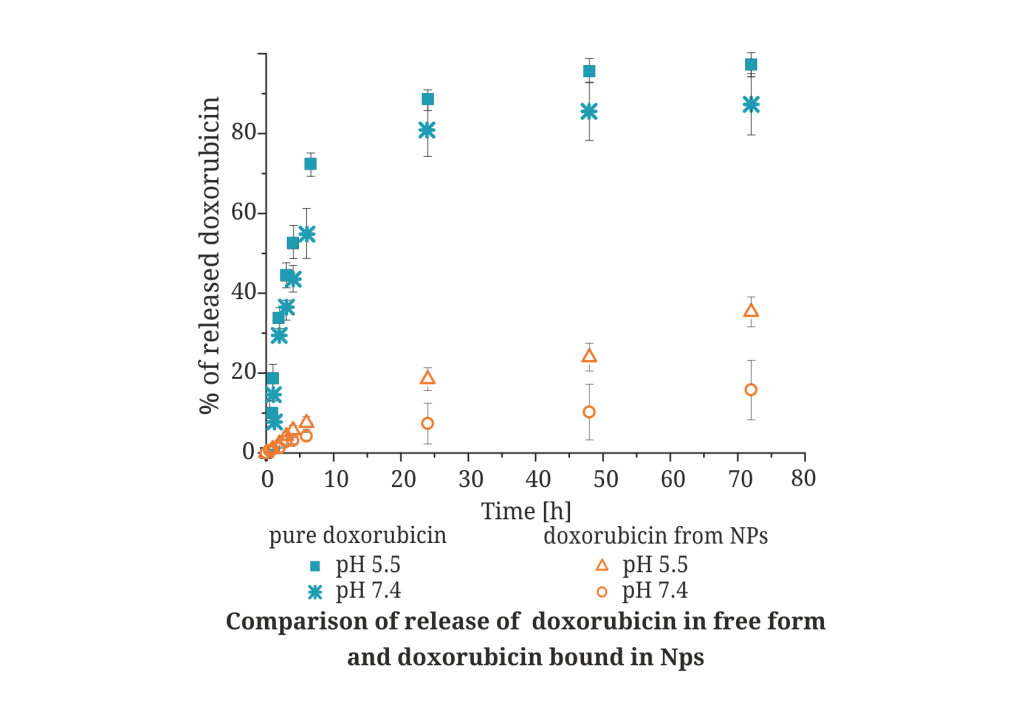
Controlled drug release |
 |


